Among the new generation of technological tools being developed to combat malaria, there is a lot of buzz around gene drives. This is a method for genetically modifying malaria-spreading mosquitoes and ultimately reducing or replacing their populations.
But how exactly do gene drives work? And how can genomic surveillance data produced by the MalariaGEN community help gene drive researchers achieve their goals safely and effectively?
What are gene drives?
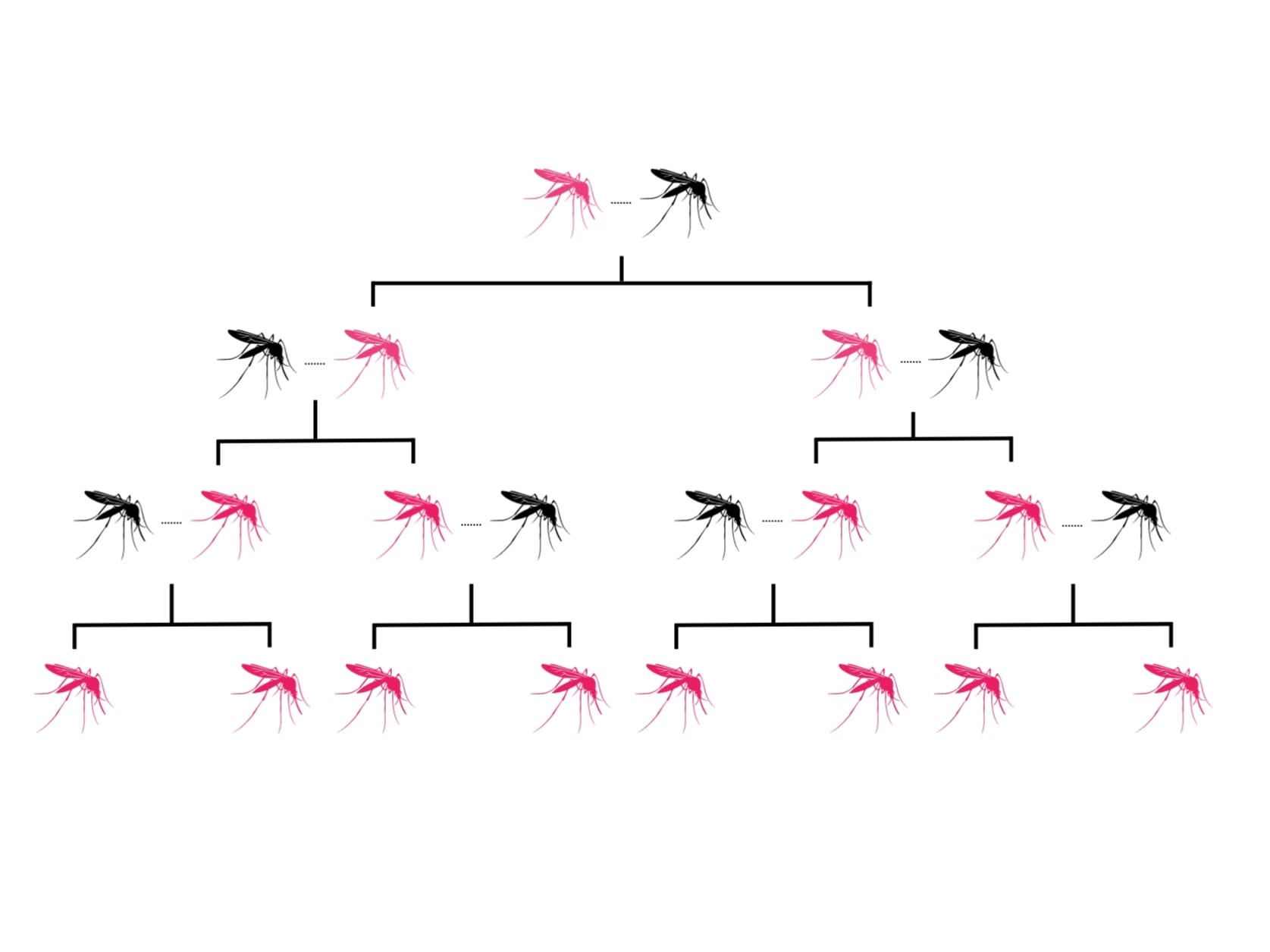
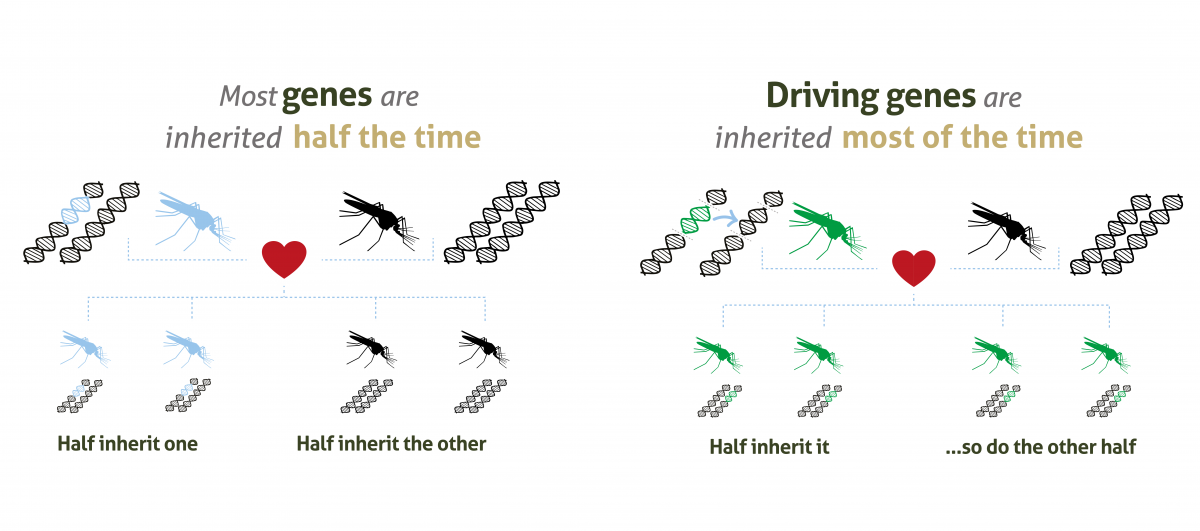
Gene drives are a ‘selfish genetic element’, in the form of lengths of DNA code, that increase the chance of a selected trait being inherited.
In normal circumstances, mosquitoes (like humans) get two copies of each gene, one from each parent. There is then an equal chance of passing either on. But when gene drive systems are present in the genome, that chance increases to up to 99%.
Gene drives do this by cutting the chromosome at the point where the desired version of the gene is absent, then inducing the cell to repair the cut with a template provided by the gene drive. The cell now has two identical copies of the same gene, ensuring the inheritance of the gene and its corresponding trait. The gene drive itself will also be inherited as part of this process.
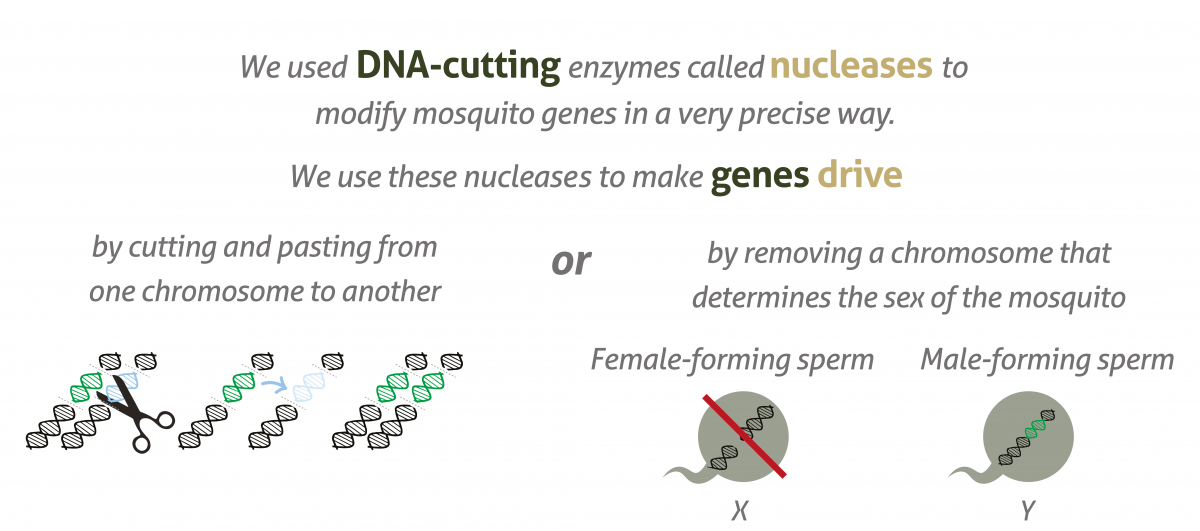
Gene drives do occur naturally, but scientists have now developed tools to insert them artificially into organisms’ genetic code by using DNA-cutting enzymes, for example CRISPR/Cas9.
Projects such as Target Malaria propose to use genetically modified mosquitoes carrying gene drives that boost the inheritance of traits affecting the reproductive genes in a way that reduces the population of malaria mosquitoes. The team is investigating two traits in particular. One gene drive causes female infertility, and another causes mosquitoes to only produce male offspring. The purpose for both is to reduce the targeted mosquito population enough to stop the transmission of malaria.
The ethical debate: Should gene drives be used, and will regulators allow it?
Can gene drives jump from one species to harm another? Will animals that eat the Anopheles species being removed from the ecosystem be adversely affected or simply switch to other prey? Ecological studies are underway to investigate these and other consequences of introducing gene drives into wild populations.

But compare gene drives to insecticides, which have the potential to harm many different organisms. Gene drives are precision tools capable of targeting just one species – which is ideal because while there are more than 3,500 species of mosquito in the world, only about 30 of those can spread malaria to humans.
Nevertheless, it is important to raise and address these concerns. Target Malaria has strict policies on evaluating and assessing the risks of their technology. Local communities are also brought into decision making, and national authorities regulate the research and use of gene drives. Recently, momentum has gathered behind gene drive – for example, with the African Union committing to engage with gene drive developers in future.
How does genomic surveillance support gene drive technology?
There are three main ways genomic surveillance can support the introduction of gene drive technologies, helping ensure they are safe and effective.
1. Will gene drives work on all the mosquitoes being targeted?
Even within each Anopheles species, there is genetic variation between individual insects. This is precisely what genomic surveillance is monitoring already, for example when we look for changes to genes which cause insecticide resistance.
When developing gene drives, researchers want to make sure that the part of the genome they are targeting is relatively similar and stable across the species. A gene drive introduced into the wild population will have limited effect if the genetic sequence that it has been designed for only appears in a small percentage of individuals. Also, if the area of the genome being targeted is seen to change over time, that may render the gene drive ineffectual in the future.
On that last concern, studies by a Target Malaria team in Burkina Faso have recently shown a reassuring stability over time in a gene being targeted for gene drives.
2. How and where do mosquitoes travel?
Individual Anopheles mosquitoes have short lifespans and don’t travel long distances. This behaviour in genetically modified mosquitoes is already being studied by Target Malaria in Burkina Faso.
But over generations, genetically related mosquitoes can spread many kilometres, forming connected populations. However, sometimes geography, climate, or other factors get in the way. That means not all mosquitoes of the same species are able to breed with one another, even across the same landmass. In these circumstances, the spread of gene drives would be limited.
Genomic surveillance can spot distinct populations by looking at genetic differences which show how genes are flowing between groups. If breeding is not taking place, separate release strategies for gene drive mosquitoes would have to be used to target both groups.
3. How would we monitor the spread of gene drives?
No gene drive mosquitoes have been released in the wild yet. Should gene drives be released into the wild in the future, it would be important to know whether they are spreading as expected. This is important for checking the strategy is working, but there are other considerations too. For example, regulatory authorities and communities may want to know whether mosquitoes carrying gene drives are present in their areas.
Genomic surveillance is one tool used for this. For example, malaria transmission may decline in an area, but how would we know this is because of gene drives? Researchers need to know gene drive mosquitoes are present. Other things might also be happening with the malaria parasite itself, or other Anopheles species in the area. Genomic surveillance can monitor these other changes too.
If gene drives are to be used effectively and safely one day – and then continued to be used for other locations, species, or even diseases – then scientists need evidence of its impact. Genomic surveillance would play a critical role in providing that evidence in the future.
To learn more about Target Malaria’s work with gene drives, visit the website. You may also be interested in the African Union’s report on gene drive for malaria control.

