
In Ethiopia, malaria transmission varies according to the season; short and long periods of rain mean that malaria-carrying mosquitoes breed during different times of the year. However, in some areas of the country where mosquitoes breed around water bodies, malaria transmission is year-round. As a result, controlling malaria and its vector requires not just new tools and approaches, but also the ability to adapt to different local contexts. A dedicated team in Ethiopia led by Dr Lemu Golassa, Head of the Medical Parasitology Research Unit at Aklilu Lemma Institute of Pathobiology, Addis Ababa University, is working tirelessly to make adaptable vector control a reality. In a recent interview, Dr Golassa shared insights from an exciting new project on malaria vector genomic surveillance and the transformative potential of genomics in combating malaria.
The story so far
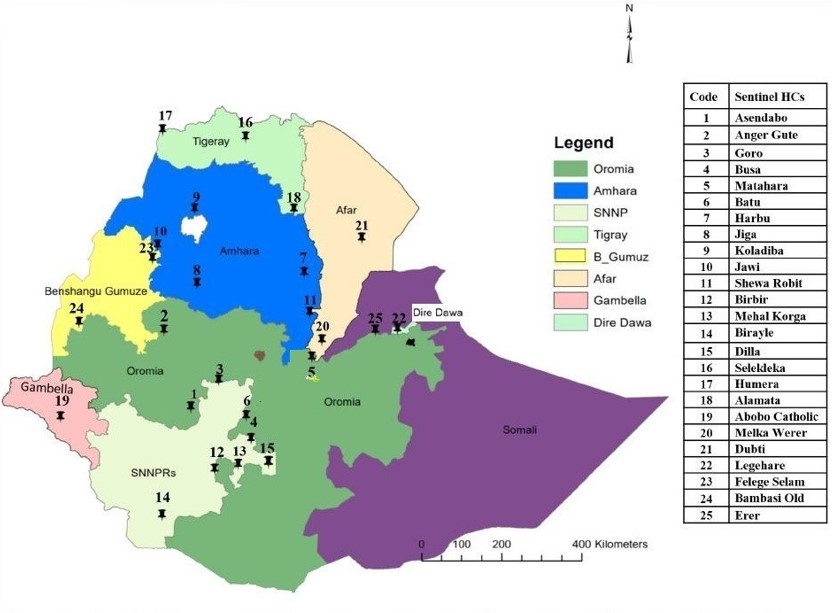
The project, funded by the Bill & Melinda Gates Foundation, kicked off in 2022. It aims to provide a comprehensive overview of insecticide resistance and malaria transmission dynamics in Ethiopia, and generate insights that can be used by the National Malaria Control Programme and other public health bodies to make informed decisions and implement effective control measures. With vector samples being collected from 10 National Malaria Sentinel Surveillance Sites representing all major malaria-endemic regions of Ethiopia, it will be one of the most comprehensive projects yet to carry out malaria vector genomic surveillance in the country.
“For more than a decade now, we have been collaborating with many African scientists to leverage malaria genomics and find the solution to eliminate malaria from the continent,” said Dr Golassa, who has been working on malaria genetics in Ethiopia for more than 12 years. “No studies have been done on systematic collection of mosquitoes from the entire country. So the hope in this project is to give us a good picture of the dynamics and evolution of insecticide resistance in the vector population in different regions of the country, that could also give us a brief overview of malaria transmission dynamics.”
By the end of the project in 2026, the team aims to generate whole genome sequence (WGS) data for more than 4,000 Anopheles arabiensis mosquitoes. The sequencing will be carried out at the Genomic Surveillance Unit at the Wellcome Sanger Institute in the UK. The team are particularly looking for the frequency of genes associated with resistance to commonly-used insecticides such as DDT and pyrethroids. They will also be paying attention to a particular genetic marker — 2La chromosomal inversions — within the Anopheles gambiae population. This marker has been linked with higher survival rates of adult mosquitoes in different microclimatic conditions such as warmer and more arid environments, which in turn affects where they can transmit malaria.
The project will yield another valuable outcome, as molecular biology experts trained for this project will be able to apply the skills and knowledge gained to explore a range of other vector-borne diseases. The data generated will also be shared, communicated, and made accessible to a global network of organisations and researchers through MalariaGEN.
On the move towards genomic tools
Malaria research has made some remarkable progress over recent years, with new vaccines, therapeutics, and vector control measures. But adoption of these innovations has been slow, especially in places where they are needed most. “Science is actually going pretty slowly in Africa,” says Dr Golassa. “We’re still using very classical and traditional methods to combat infectious diseases, including malaria. While science has been developing elsewhere, [here in Ethiopia,] we aren’t really accustomed to using these new scientific advancements and genomic data to support malaria elimination efforts.”
Classical methods for malaria entomological surveillance have long been the primary approach in Ethiopia, but they have their limitations. Dr Golassa cited one such example, phenotypic assays, which can be used to assess a mosquito population’s susceptibility to an insecticide in laboratory conditions. However, these methods have their limitations. “[Classical methods] are not informative in real time, and we know little about how pathogens and vectors have been changing,” he explained. These methods can only assess established markers of resistance identified in specific regions of the genome, so other potential markers operating in synergetic ways with those established markers would be missed out.
He stressed the urgency of embracing genomics to gain a deeper understanding of the genetic aspects of malaria parasites and vectors over time. “We have to move with the science in order to tackle the evolving drug resistance in the malaria parasite and insecticide resistance in the vector. Using genomic tools, we can zoom into entire regions of the genome and look at SNPs in other regions of the genome that could contribute to insecticide resistance along with the established markers of resistance. This would help us map regions in the genome [that are] under selection pressure.”

Ethiopia’s malaria calendar
To lay the groundwork for a project like this, it is crucial to recognise a seemingly trivial yet influential fact: mosquitoes need a wet environment to breed. During the rainy seasons, mosquito eggs and larvae could be washed away before they have a chance to grow into the adult stages, so the ideal times for mosquito breeding are just after the rainy seasons end. This is when Dr Golassa’s team collects the mosquitoes. But this may not be the same time throughout the country.
Ethiopia is a large, geographically diverse country with a gradient of malaria transmission. As a result, the team needs to account for variations in rainy season patterns in different regions, so that sample collection is planned based on when mosquito populations breed in each location. Dr Golassa spoke about how his team plans for this: “We conduct mosquito collections during two seasons. The first is the minor malaria transmission season, which occurs from April to May following the short rainy season from February to March. The second transmission season is from September to December, which is after the main rainy season from June to August. After this extended rainy season, we observe a large population of mosquitoes involved in malaria transmission.”
So far, the team has collected over 400 samples from five different sentinel sites up until the minor transmission season of 2023. Next, they will shift focus to the impending major transmission season. “This will enable us to conduct a comprehensive study on the mosquito population dynamics and the resistance of insecticides during both seasons.”
The biting consequences of changing behaviour and climates
Malaria risk typically peaks after rainy seasons in Ethiopia, but Dr Golassa’s team has observed recent changes to these patterns. He explains that changing land use patterns and climate change are altering the local temperatures and humidities enough that malaria and mosquito territories are changing these days.
Because mosquito breeding patterns are affected by their micro-environment, Dr Golassa also reflected on the role that ever-changing human activities play: “Human land use activities for agriculture and irrigation — or schemes for electricity — would modify the natural environment, making it ideal for mosquito breeding. There are instances where human activities have turned non-endemic areas of Ethiopia to become malaria endemic. For instance, irrigation and dam projects have been expanding in recent years and this favours malaria transmission. Apart from this, people’s mobility from one region to another for agricultural activities could challenge malaria control efforts.”
Dr Golassa is, however, hopeful that the move towards genomic surveillance methods will help track the malaria vector despite these changes. “Indeed, genomic tools can also help us explore vector populations that are better adapted to changes in climates and keep evolving using their genomic elasticity.”
Overcoming data gaps
Dr Golassa provided some glimpses into how his team usually prepares for sample collection based on established MalariaGEN mosquito collection guidance. “Before we embark on mosquito collection from the field, we should first go to the local administrative office and get permission. Once permission is obtained, together with the local health professionals, we identify households and other areas suitable for adult mosquito collection. Of course this will influence the types of tools we will use for mosquito collection. Sometimes, animal shelters could also be considered for mosquito collection. Once the sites are identified, then we will place the collection devices accordingly. In the morning, we check the number of mosquitoes trapped.”
While these activities follow a standard set of protocols and procedures, there is seldom a one-size-fits-all approach to implementing a project because each context is unique, and sometimes it involves navigating the unpredictable. In 2020, there was an outbreak of armed conflict in the Tigray region of Ethiopia, with its effects felt around the country in many ways. For Dr Golassa’s team, this meant that some regions of the country where sample collection had been planned became difficult to access. He highlighted the unpredictability of such circumstances, which can disrupt planned visits to specific areas. “Because there were some security issues in the country, we were advised not to travel to some localities. When you write a proposal, the funny thing is, everything looks normal, but all of a sudden, something will happen in the region amidst sample collection. And then you are forced to wait for some time until the situation becomes normal.”
However, he remains optimistic that stability will be restored, allowing his team to reach all sentinel sites and gather vital data needed for a comprehensive understanding of malaria vector dynamics. “The hope in this project is to give us a good picture of vector dynamics in the country, so our aim is to visit all those sentinel sites when things get to normal.”
One down, three to go
As the project moves into its second year, there is little doubt that it holds great promise in improving our understanding of malaria transmission dynamics in Ethiopia and ultimately contributing to the elimination of this devastating disease. Despite challenges faced in the Ethiopian context, Dr Golassa and his team demonstrate that building local genomic surveillance capacity for malaria is worth investing in, especially at a time when changing climates and human behaviour render classical tools ineffective. Most of all, the project is an inspiring example of what is possible through global collaboration, data and resource sharing, and the unwavering efforts of a dedicated team.

